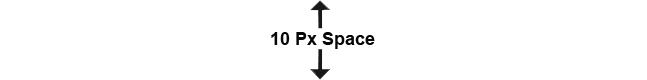
Our production department is at the heart of our cGMP API manufacturing operations, dedicated to producing high-quality hormone active pharmaceutical ingredients (APIs) that meet the stringent regulatory standards.
Our manufacturing facility consists of three dedicated hormone production plants, each with technical area for intermediate synthesis and clean area for final API synthesis and powder processing. Clean areas are classified Grade D areas. Our production areas are designed to minimize contamination risks, featuring controlled environments that adhere to strict temperature and humidity regulations. Synthesis and powder processing is done taking utmost precautions in close conditions and use of air pressure suits by production personnel to avoid product exposure.
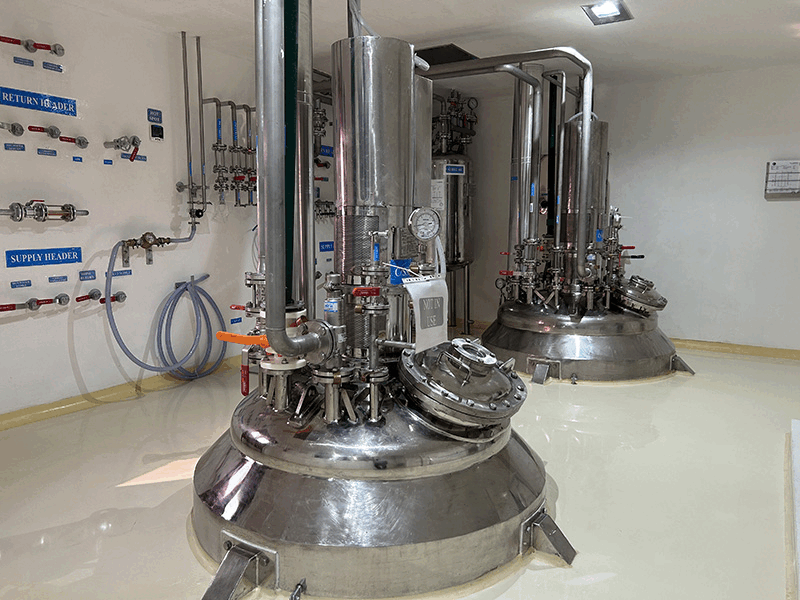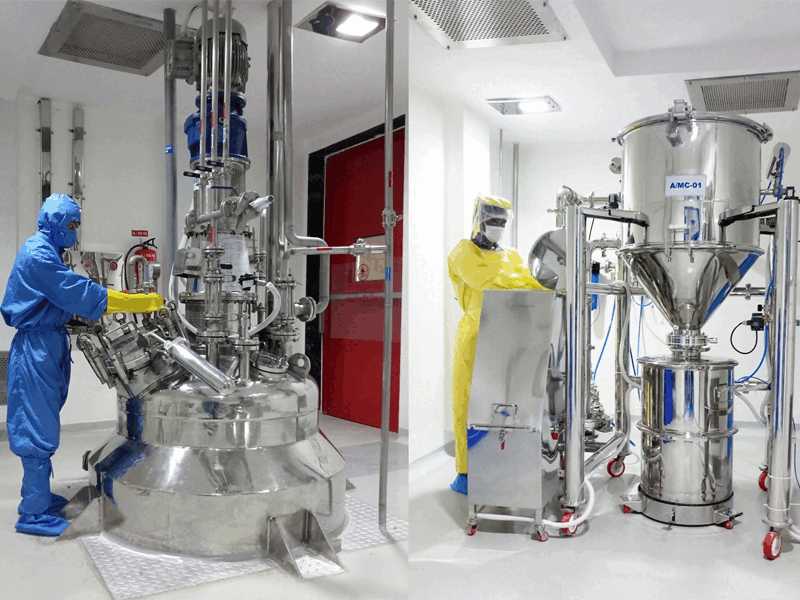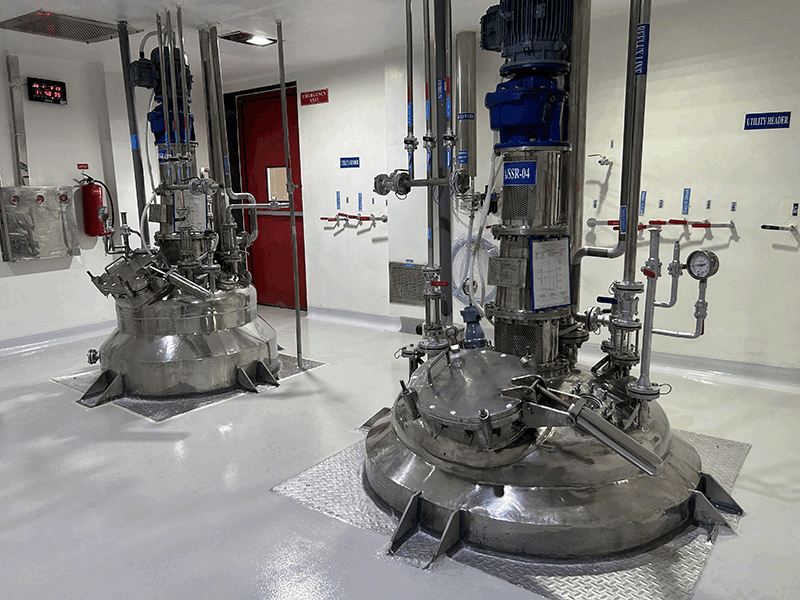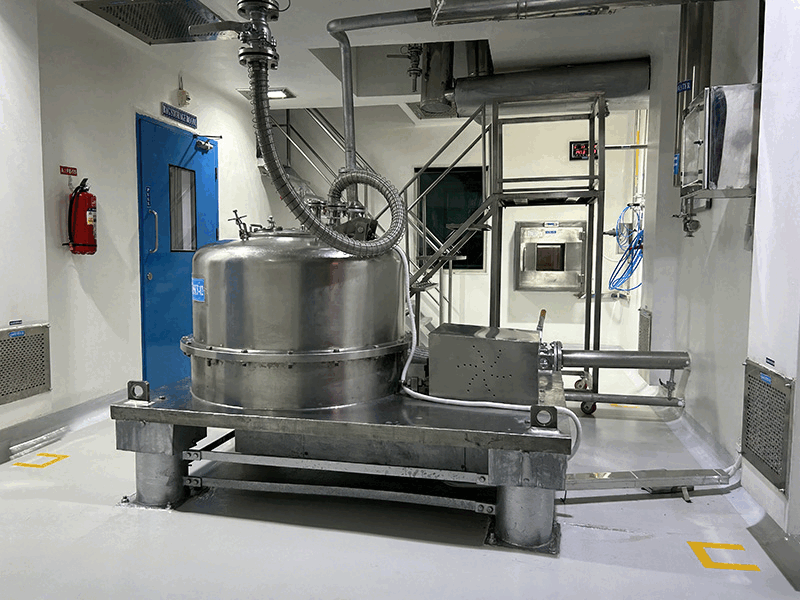
We employ a robust multi-step synthesis process, utilizing the latest technologies and equipment to convert raw materials into high-purity APIs. Each synthesis block is equipped with advanced reactors, agitators, and separation technologies tailored to specific chemical processes meeting cGMP requirements.
Our production lines are designed for flexibility and scalability, accommodating small-scale batches for developmentin pilot plant and larger volumes having a total production volume of over 30,000 litres for commercial supply. This adaptability allows us to meet diverse customer needs efficiently.
714, Maple Trade Centre, Near Surdhara Circle, Thaltej, Ahmedabad-380054, Gujarat - India
[email protected]
[email protected] (For product inquiry)
Copyright © 2025 La Chandra Pharmalab All rights reserved.