Our Quality Assurance and Quality Systems reflect our unwavering commitment to delivering high-quality APIs that meet the needs of our customers and compliance with current Good Manufacturing Practices (cGMP) as mandated by leading global regulatory authorities including:
By fostering a culture of adherence to quality systems and continuous improvement, we strive to exceed expectations of our customers worldwide.
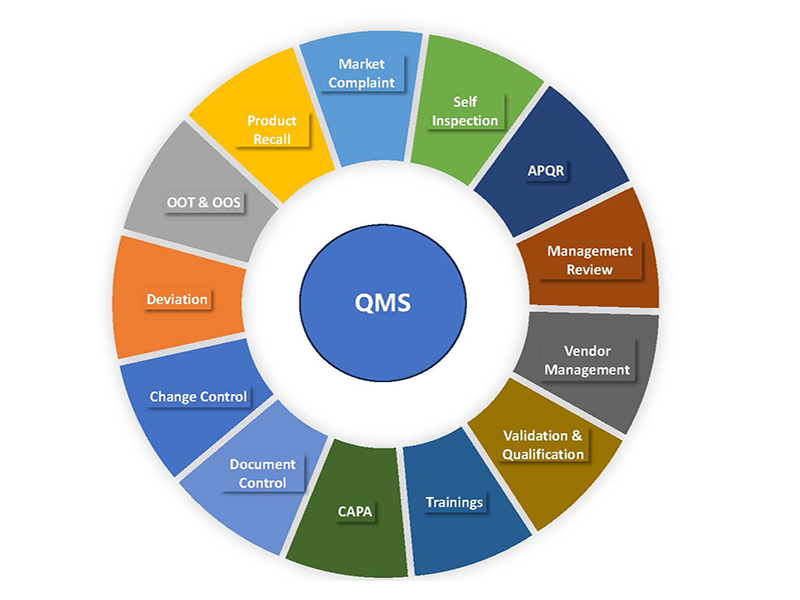
Our QMS integrates all quality-related processes, from research and development to manufacturing and distribution. This holistic approach ensures consistency and compliance across all departments.
Regular internal audits are conducted to assess compliance with our QMS and identify areas for improvement. These audits help ensure that we uphold our commitment to quality at all levels of the organization.
We maintain meticulous documentation for all quality-related activities, including batch records, testing results, OOS and deviation reports. This ensures traceability and provides a robust foundation for audits and inspections.
Detailed SOPs govern every aspect of our operations, ensuring that all processes are conducted consistently, effectively and in accordance with cGMP guidelines. These procedures are regularly reviewed and updated to incorporate best practices and regulatory changes.
We implement strict quality assessments for all suppliers to ensure that raw materials meet our high standards. Regular evaluations and audits of our suppliers help to maintain the integrity of our supply chain.
A formal change control process is in place to assess and document any changes to processes, equipment, or materials. This ensures that all changes are thoroughly evaluated for their impact on quality and compliance.
We invest in continuous training for our employees to ensure they are knowledgeable about quality standards, procedures, and regulatory requirements. This commitment to training enhances our overall quality culture and knowledgeable workforce.
We employ a proactive approach to risk management, identifying potential risks to quality and implementing mitigation strategies to minimize their impact. This includes regular risk assessments and analysis of critical processes.
Finished product labels affixed on each container of API has quick response code (QR code) as prescribed in gazette notification issue by the Ministry of Health and Family Welfare to facilitate tracking and tracing of the products in the supply chain.
Our commitment to continuous improvement and quality excellence is reinforced through systematic management reviews. These reviews are a critical component of our Quality Management System (QMS) and serve to evaluate the effectiveness of our quality processes, ensure alignment with strategic goals, and drive ongoing enhancements.
714, Maple Trade Centre, Near Surdhara Circle, Thaltej, Ahmedabad-380054, Gujarat - India
[email protected]
[email protected] (For product inquiry)
Copyright © 2025 La Chandra Pharmalab All rights reserved.