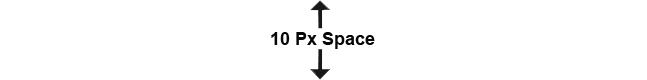
Stringent Quality control is a cornerstone of our operations and La Chandra has a well-equipped QC laboratory with instruments set-up under a network data system, with complementary wet-lab and microbiology units. Our overall quality standards are monitored and regularly updated via training programs and internal audits.
All incoming raw materials undergo thorough testing to verify their quality and compliance with specifications before they are approved for use in our manufacturing processes. In-Process control for continuous monitoring and testing during production ensure that processes remain within defined parameters, minimizing variability and ensuring product consistency. Each batch of finished API undergoes extensive analytical testing, including identity, potency, purity, microbiological quality and stability assessments, as per pharmacopeial requirements before release.
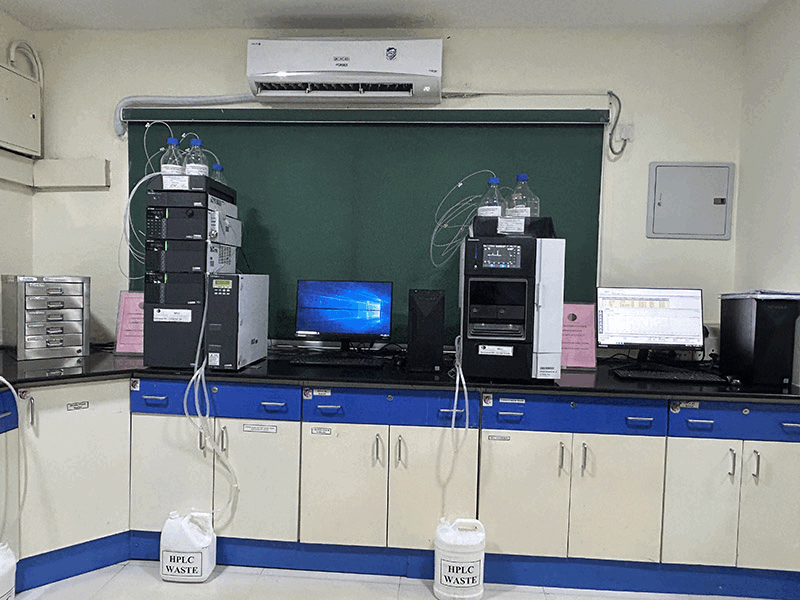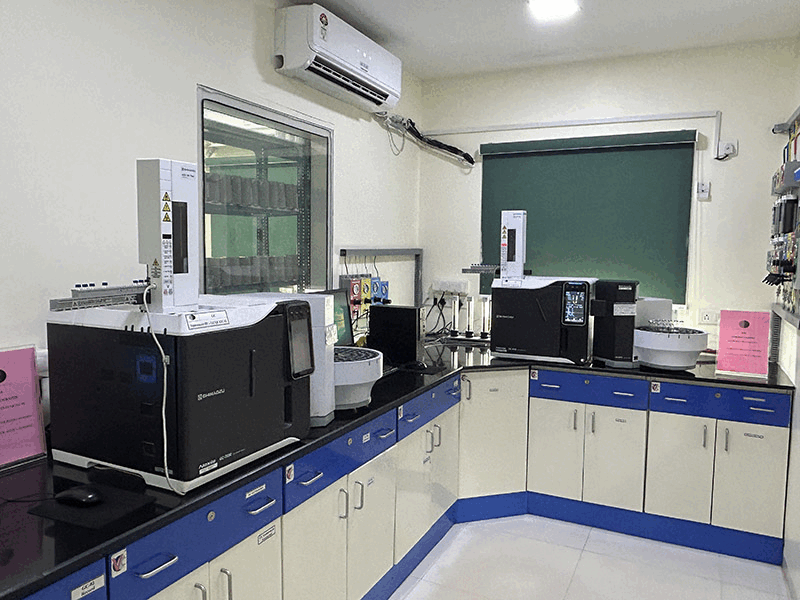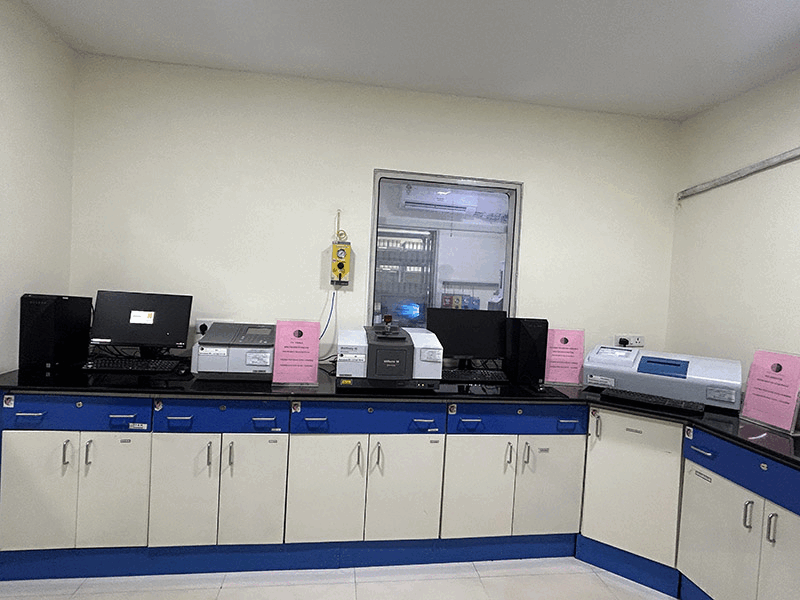
We conduct stability studies to evaluate the shelf life and storage conditions of our APIs, ensuring that our products maintain their quality over time and under various environmental conditions including Zone IVb as per ICH Q1 guidelines.
Our QC departement is well equipped with advanced instrumentation to enhance the accuracy, efficiency, and reliability of our Quality Control (QC) processes. These are 21 CFR Part 11 compliant, qualified and periodically calibrated. Computerised systems have been validated (CSV).
714, Maple Trade Centre, Near Surdhara Circle, Thaltej, Ahmedabad-380054, Gujarat - India
[email protected]
[email protected] (For product inquiry)
Copyright © 2025 La Chandra Pharmalab All rights reserved.